Air Composition and Molecular Weight

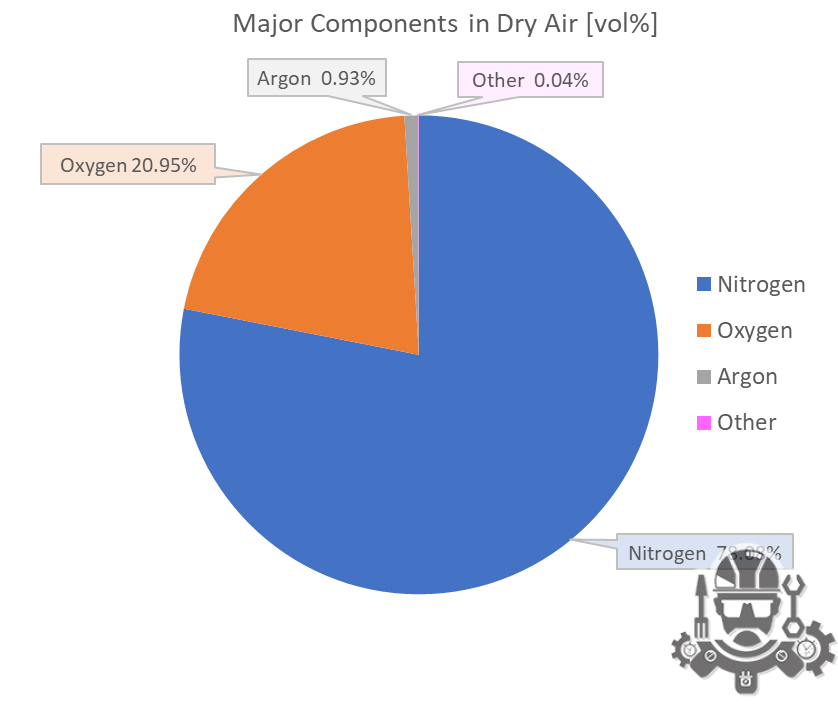
Air is a mixture of gases that primarily consists of nitrogen, oxygen, carbon dioxide, and traces of other gases. Air composition of air can vary slightly depending on factors such as location, altitude, and pollution levels. Here is the approximate composition of dry air:
- Nitrogen (N2): Nitrogen makes up the largest portion of dry air, accounting for about 78% of the total volume.
- Oxygen (O2): Oxygen is the second most abundant gas in air, comprising approximately 21% of the total volume.
- Carbon Dioxide (CO2): Carbon dioxide is present in trace amounts, making up around 0.04% of the total volume.
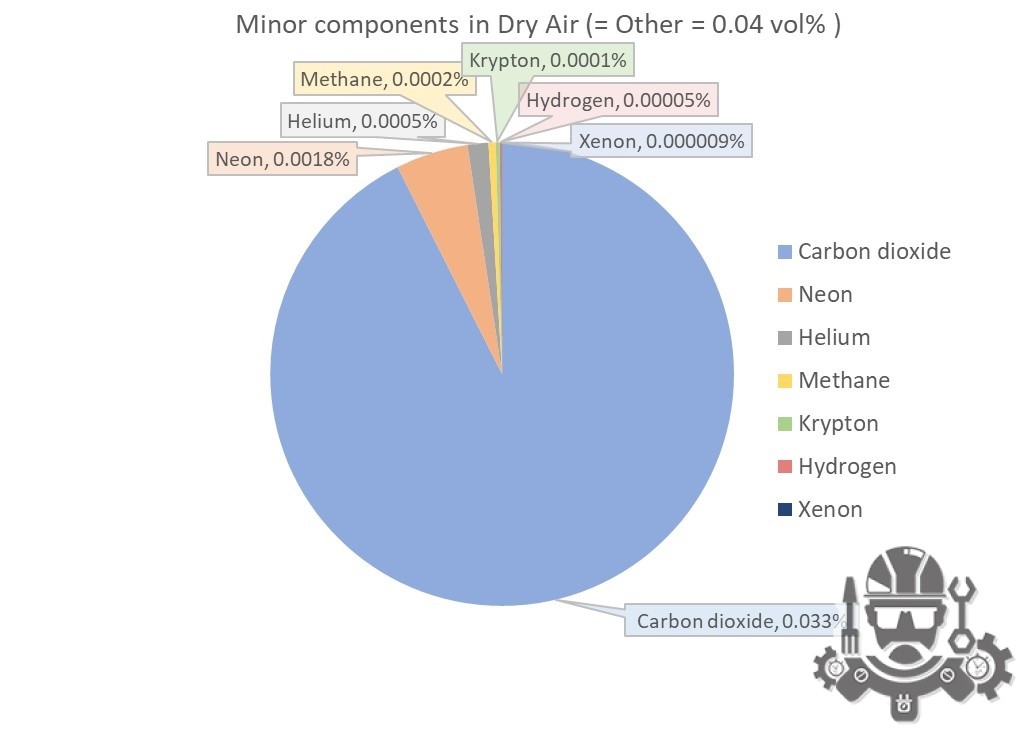
- Other Gases: Air also contains small amounts of other gases, including argon (0.93%), neon, helium, methane, krypton, and xenon, as well as water vapor, which can range from near 0% in extremely dry conditions to around 4% in humid environments.
The molecular weight of air is the average of the molecular weights of its constituent gases. The molecular weight of dry air is approximately 28.97 grams per mole (g/mol). This value is calculated by considering the molecular weights and relative proportions of nitrogen, oxygen, carbon dioxide, and other trace gases in air.
It’s worth noting that the composition of air can vary slightly in different parts of the world or under specific conditions. For example, areas with high levels of pollution may have increased levels of pollutants in the air. Additionally, at higher altitudes, the concentration of oxygen may decrease due to lower atmospheric pressure.
Contents
Air Composition
Air is usually modeled as a uniform (no variation or fluctuation) gas with properties averaged from the individual components.
| Components in dry air | Volume ratio = Molar ratio compared to dry air |
Molar mass | Molar mass in air | Atmospheric boiling point |
|||||
| Name | Formula | [mol/molair] | [vol%] | [g/mol], [kg/kmol] |
[g/molair], [kg/kmolair] |
[wt%] | [K] | [°C] | [°F] |
| Nitrogen | N2 | 0.78084 | 78.084 | 28.013 | 21.872266 | 75.511 | 77.4 | -195.8 | -320.4 |
| Oxygen | O2 | 0.20946 | 20.946 | 31.999 | 6.701942 | 23.14 | 90.2 | -183.0 | -297.3 |
| Argon | Ar | 0.00934 | 0.934 | 39.948 | 0.373025 | 1.29 | 87.3 | -185.8 | -302.5 |
| Carbon dioxide1) | CO2 | 0.000412 | 0.0412 | 44.010 | 0.018132 | 0.063 | 194.7 | -78.5 | -109.2 |
| Neon | Ne | 0.00001818 | 0.001818 | 20.180 | 0.000367 | 0.0013 | 27.2 | -246.0 | -410.7 |
| Helium | He | 0.00000524 | 0.000524 | 4.003 | 0.000021 | 0.00007 | 4.2 | -269.0 | -452.1 |
| Methane | CH4 | 0.00000179 | 0.000179 | 16.042 | 0.000029 | 0.00010 | 111.7 | -161.5 | -258.7 |
| Krypton | Kr | 0.0000010 | 0.0001 | 83.798 | 0.000084 | 0.00029 | 119.8 | -153.4 | -244.0 |
| Hydrogen | H2 | 0.0000005 | 0.00005 | 2.016 | 0.000001 | 0.000003 | 20.3 | -252.9 | -423.1 |
| Xenon | Xe | 0.00000009 | 0.000009 | 131.293 | 0.000012 | 0.00004 | 165.1 | -108.1 | -162.5 |
| Average molar mass of air | 28.9647 | ||||||||
1) According NASA CO2 level in 1960 aprox. 320 ppm, 1970 aprox. 328 ppm, 1980 aprox. 341 ppm, 1990 aprox. 356 ppm, 2000 aprox. 372 ppm, 2010 aprox. 390 ppm and 2020 aprox. 412 ppm
Other components in air
- Sulfur dioxide – SO2 – 1.0 parts/million (ppm)
- Nitrous oxide – N2O – 0.5 parts/million (ppm)
- Ozone – O3 – 0 to 0.07 parts/million (ppm)
- Nitrogen dioxide – NO2 – 0.02 parts/million (ppm)
- Iodine – I2 – 0.01 parts/million (ppm)
- Carbon monoxide – CO – 0 to trace (ppm)
- Ammonia – NH3 – 0 to trace (ppm)
Common Pressure Units frequently used as alternative to “one Atmosphere”
- 76 Centimeters (760 mm) of Mercury
- 29.921 Inches of Mercury
- 10.332 Meters of Water
- 406.78 Inches of Water
- 33.899 Feet of Water
- 14.696 Pound-Force per Square Inch
- 2116.2 Pounds-Force per Square Foot
- 1.033 Kilograms-Force per Square Centimeter
- 101.33 kiloPascal