Air – Thermophysical Properties

Air is a mixture of gases, primarily consisting of nitrogen (about 78% by volume) and oxygen (about 21% by volume), with trace amounts of other gases such as argon, carbon dioxide, and various inert gases. The thermophysical properties of air vary with temperature and pressure. Here are some important thermophysical properties of dry air at standard atmospheric pressure (101.325 kPa or 1 atm):
- Density: The density of dry air at standard conditions is approximately 1.225 kg/m³.
- Specific Heat Capacity: The specific heat capacity of dry air at constant pressure (Cp) is around 1005 J/(kg·K), and at constant volume (Cv) is around 717 J/(kg·K). The ratio of specific heat (Cp/Cv) is approximately 1.4.
- Thermal Conductivity: The thermal conductivity of air at room temperature is about 0.0257 W/(m·K). It increases with temperature.
- Viscosity: The dynamic viscosity of air at room temperature is about 1.8 × 10^(-5) Pa·s.
- Speed of Sound: The speed of sound in air at room temperature is approximately 343 meters per second.
- Saturation Pressure: The saturation pressure of water vapor in air depends on temperature. At 20 degrees Celsius, the saturation pressure is around 2.34 kPa.
- Humidity: The humidity of air is expressed in terms of relative humidity (RH), which is the ratio of the partial pressure of water vapor to the saturation pressure at a given temperature. Absolute humidity is the mass of water vapor per unit volume of air.
It’s important to note that these values are approximate and can vary based on the specific composition of air, especially concerning water vapor content, which can significantly influence properties like density and specific heat capacity. Additionally, these values may change at different altitudes and temperatures, so specific conditions should be considered when calculating or using these properties.
Thermophysical properties of air
- Boiling temperature (at 1 bara): 78.8 K = -194.4 °C = -317.8 °F
- Bulk modulus elasticity : 1.01325 x 10 5 Pa or N/m 2
- Condensation temperature (at 1 bara): 81.8 K = -191.4 °C = -312.5 °F
- Critical temperature : 132.63 K = -140.52 °C = -220.94 °F
- Critical pressure : 37.363 atm = 37.858 bar = 3.7858 MPa (MN/m 2 ) = 549.08 psi (=lb f /in 2 )
- Critical density: 10.448 mol/dm 3 = 302.6 kg/m 3 = 0.5871 slug/ft 3 = 18.89 lb m /ft 3
- Density (at 0°C and 1 bara): 1.276 kg/m 3 = 0.00248 slug/ft 3 = 0.0797 lb/ft 3
- Density (at 60°F and 1 atm): 1.208 kg/m 3 = 0.00234 slug/ft 3 = 0.0754 lb/ft 3
- Enthalpy (heat) of air at 0°C and 1 bara: 11.57 kJ/mol = 399.4 kJ/kg = 171.7 Btu(IT)/lb
- Entropy of air at 0°C and 1 bara: 0.1100 kJ/mol K = 3.796 kJ/kg K = 0.9067 Btu(IT)/lb °F
- Liquid density at boiling point and 1 bar: 875.50 kg/m 3 = 54.656 lb/ft 3
- Molar mass: 28.9647 g/mol
- Specific heat capacity (C p ) air at 0°C and 1 bara: 1.006 kJ/kgK = 0.24028 Btu(IT)/(lb m °F) or kcal/(kg K)
- Specific heat capacity (C v ) air at 0°C and 1 bara: 0.7171 kJ/kgK = 0.17128 Btu(IT)/(lb m °F) or kcal/(kg K)
- Thermal conductivity at 0°C and 1 bara: 24.35 mW/(m K) = 0.02094 kcal(IT)/(h m K) = 0.01407 Btu(IT)/(h ft °F)
- Thermal expansion coefficient at 0°C and 1 bara: 0.00369 1/K = 0.00205 1/°F
- Triple point pressure: 0.05196 atm = 0.05265 bar = 5265 Pa = 0.7636 psi (=lb f /in 2 )
- Triple point temperature: 59.75 K = -213.40 °C = -352.12 °F
- Viscosity, dynamic , at 0°C and 1 bara: 17.22 μPa s = 0.01722 cP = 0.3596x 10 -6 ( lb f s)/ft 2 = 11.57 x10 -6 lb m /(ft s)
- Viscosity, kinematic , at 0°C and 1 bara: 0.00001349 m 2 /s = 13.49 cSt = 0.0001452 ft 2 /s
Example – Mass of Air at Temperature 100 o C
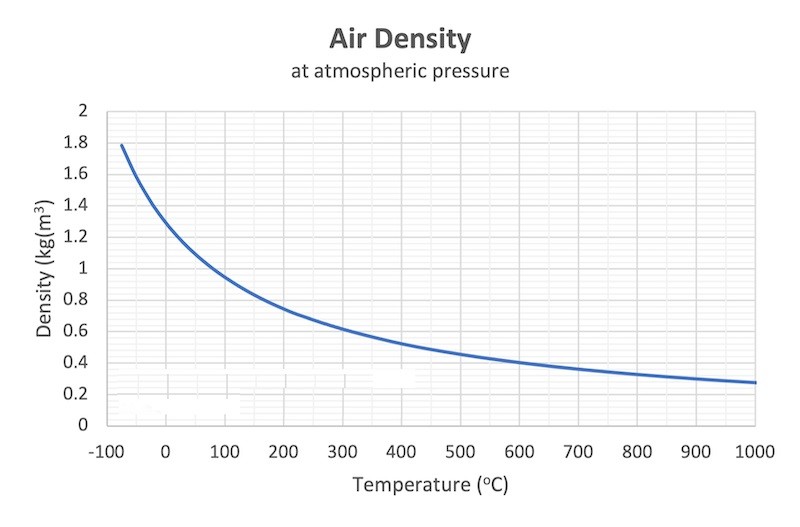
From the table above – the density of air is 0.946 kg/m 3 at 100 o C . The mass of 10 m 3 air can be calculated as
m = V ρ
= (10 m 3 ) (0.946 kg/m 3 )
= 9.46 kg
where
m = mass (kg)
V = volume (m 3 )
ρ = density (kg/m 3 )
Example – Mass of Air at Temperature 20 o C
From the table above – the density of air is 1.205 kg/m 3 at 20 o C . The mass of 10 m 3 air can be calculated as
m = (10 m 3 ) (1.205 kg/m 3 )
= 12.05 kg
Example – Lifting Force of a Hot Air Balloon
An air balloon with volume 10 m 3 is heated to 100 o C . The temperature of the surrounding air is 20 o C. The change in gravity force (weight) of the air volume is the potential lifting force of the balloon. The lifting force can be calculated as
F l = dm a g
= V d ρ a g
= (10 m 3 ) [(1.205 kg/m 3 ) – (0.946 kg/m 3 )] (9.81 m/s 2 )
= 25.4 N
where
F l = lifting force – change in gravity force (weight) (N)
a g = acceleration of gravity (9.81 m/s 2 )
dm = V d ρ = change of mass in the balloon (kg)
dρ = change in density due to temperature difference (kg/m 3 )